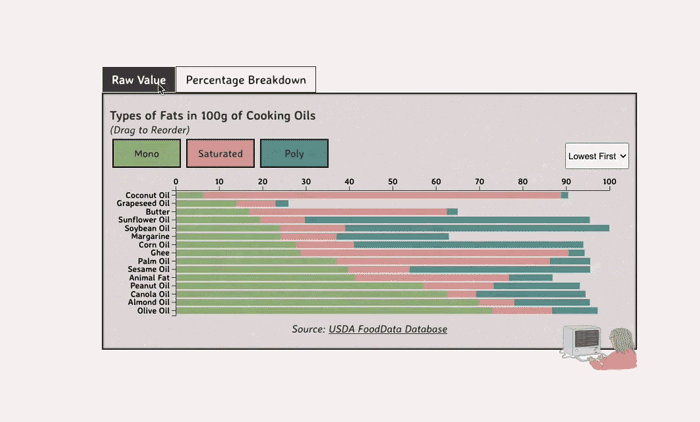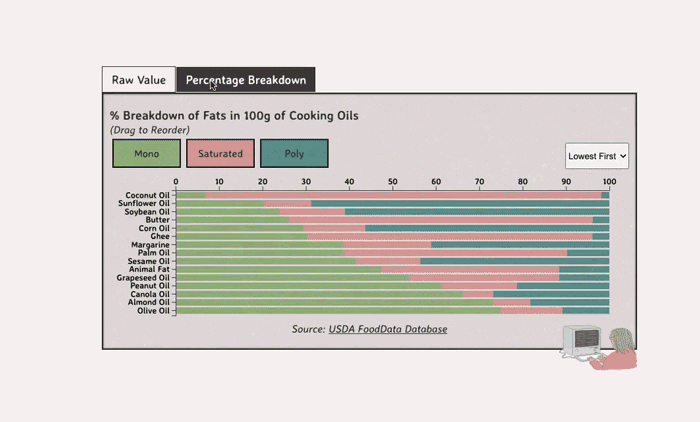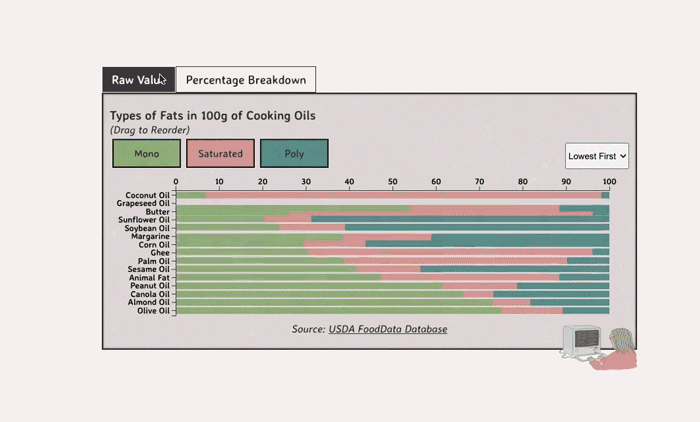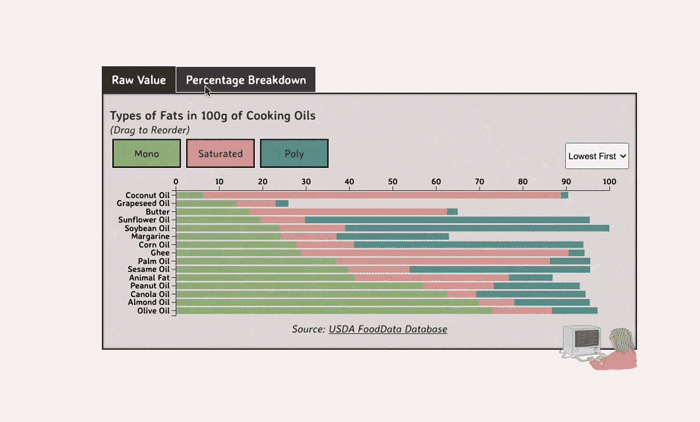
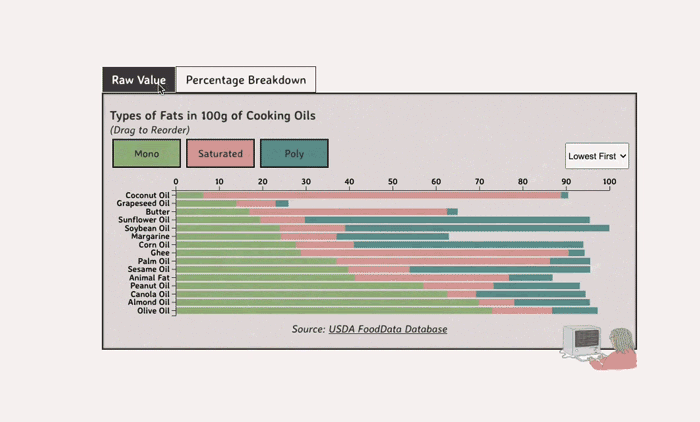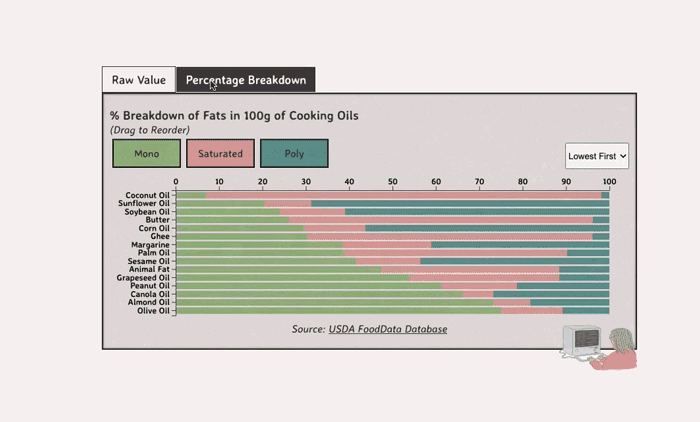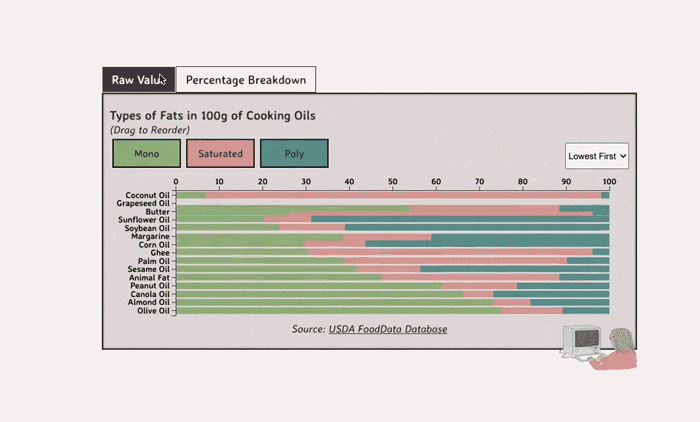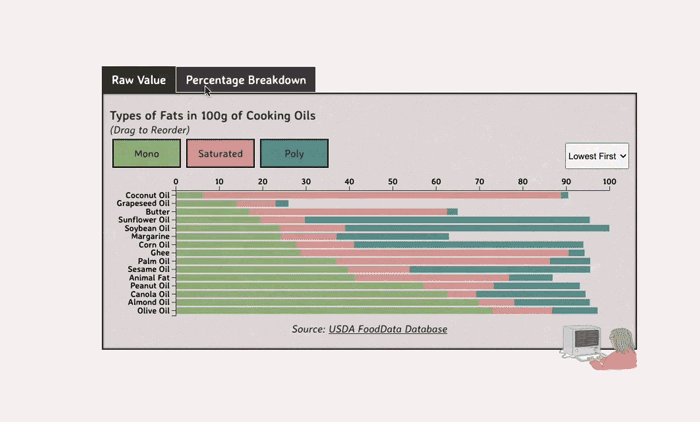
Comparing Cooking Oils, and Their Fat Content
Unsaturated fats (both poly and mono) get a slight edge over saturated because the latter adds to cholesterol levels
Gourmet Data is a blog where a data journalist breaks down questions. Subscribe either here on Ghost or on Substack.
As one of the three macronutrients we get from food (the other two being protein and carbs), fats should make up about 30% of our diet.
There are multiple types of fats: two found naturally (saturated and unsaturated), and one made artificially (trans fat). There are probably important differences in their chemistry, but I really only care about the nutritional differences between the types of fats, and the tldr is:
- Saturated fats are found in animal products, and are usually solid at room temp (butter, lard, etc)
- Unsaturated fats are liquid and are found in veggies and nuts
- Trans fat both increases 'bad cholesterol' and decreases 'good cholesterol', and should be avoided
- Unsaturated fats (both poly and mono) get a slight edge over saturated because the latter adds to LDL cholesterol levels
- just because saturated fats are less healthy doesn't mean they're unhealthy
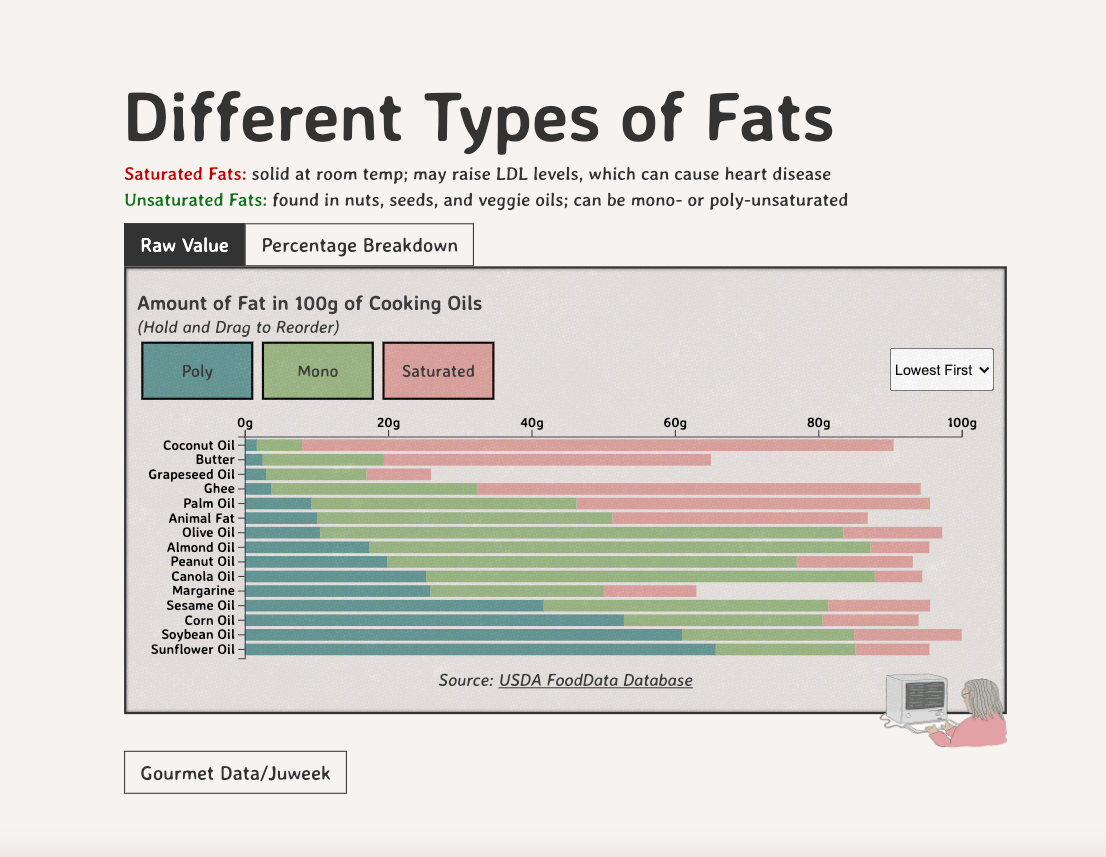
Different Types of Fats
Saturated Fats: solid at room temp; may raise LDL levels, which can cause heart disease
Unsaturated Fats: found in nuts, seeds, and veggie oils; can be mono- or poly-unsaturated
Now, on to the deep dive.
Understanding the Nutrition
The thing about fat is that they are great reserves of energy for the body. There was a 'fat-free' boom that took hold in the 80s and 90s, luckily America has mainly moved past that trend, as insufficient fat can lead to vitamin deficiencies and general bad vibes.
Saturated Fats
Saturated fats are found in high quantities in animal products and some tropical oils (coconut). Consuming too much saturated fat can elevate LDL (low-density lipoprotein) cholesterol levels. LDL is a problem because it collects in the walls of your blood vessels, heightening the risk of cardiovascular issues, like heart disease and stroke. Saturated fats also seem to raise HDL, eg good cholesterol, so it's looking bad for mans.
That said, cholesterol isn't all bad; it protects our nerves and leads to healthy cellular and hormone growth, so the key is balance. Last thing we need is another fat-free boom.
Unsaturated Fats
In contrast, unsaturated fats are usually liquid at room temperature and can be found predominantly in plants and fish. There are chemical reasons why we divide unsaturated fats into two mono and poly, but both types help reduce LDL cholesterol levels, and are considered heart-healthy fats.
When thinking of polyunsaturated fats, think of omega-6 and omega-3 fatty acids. They decrease LDL and total cholesterol 8% to 12% when compared with saturated fatty acids, and a quick google search can show you all the benefits of omega3.
Monounsaturated fats are often found in meat and dairy products as well, but also in plants. When monounsaturated fatty acids replace saturated fatty acids, LDL and total cholesterol drop by 6% to 10%.
Trans fats, funny enough, are a type of unsaturated fat created through a process called hydrogenation, where hydrogen is added to liquid vegetable oils to make them more solid. Consuming trans fats can raise levels of "bad" LDL cholesterol and lower levels of "good" HDL cholesterol, which increases the risk of heart disease and stroke
Understanding the Chemistry
Chemically speaking, Fats, in short, consist of 2 parts: an alcohol called glycerol, and 3 long chains of these fatty acids.
These fatty acid chains made up of carbon, oxygen and hydrogen atoms; they look like this, or like this (3D). Carbon atoms form the backbone of the chain, while oxygen and hydrogen atoms latch on to available slots. And this is the crux of the chemical difference between the three types of fats: how many open slots there are.
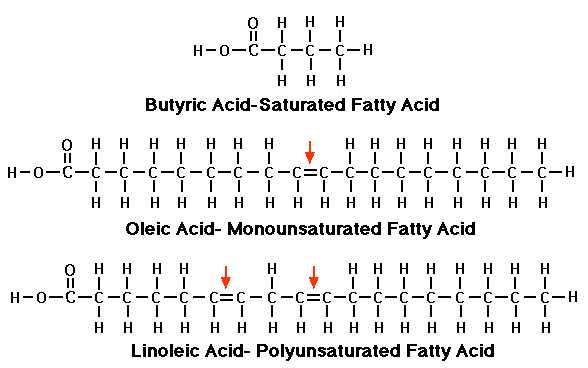
- In saturated fats, all carbon atoms in the fatty acid chains are bonded to hydrogen atoms, and there are no more open slots for oxygen or hydrogen to bond with. This results in a rigid structure that allows them to pack tightly together, hence their solid state.
- Because mono and poly unsaturated fats feature double bonds between carbon atoms, there are open slots where oxygen or hydrogen has yet to bound. This introduced 'kinks' in the molecular structure, preventing the molecules from packing tightly together, resulting in a liquid state
- Mono has one double bond, while polyunsaturated fats have multiple double bonds. Thus, monounsaturated fats has one open spot, and polyunsaturated fat has more than one open slots
- Trans fat are created through an industrial process called hydrogenation, where hydrogen is added to liquid vegetable oils to make them more solid. This process can increase the shelf life and flavor stability of foods containing these fats.